Abstract
Background: Chimeric antigen receptor modified T cells directed against CD19 (CART19) has demonstrated efficacy in relapsed or refractory (r/r) B-cell lymphoma with durable complete remissions (CR). However, there are some patients with minimal residual disease (MRD) also need attention. Persistence or reappearance of minimal residual disease (MRD) after chemotherapy always results in relapse. MRD is an indicator of resistance to chemotherapy. Based on these considerations, a clinical retrospective trials with control and without random study (ChiCTR-ONN-16008911) was conducted to determine the efficacy and safety of CD19-CAR-T cells in MRD positive B-cell lymphoma patients, which is devoted to reduce the risk of recurrence. This paper will discuss the difference of efficacy and safety between minimal residual disease or partial remission and relapsed or refractory patients. The structural features of our CAR-T products include anti-CD19 scFv, a transmembrane domain, and a 4-1BB/CD3ζsignaling domain.
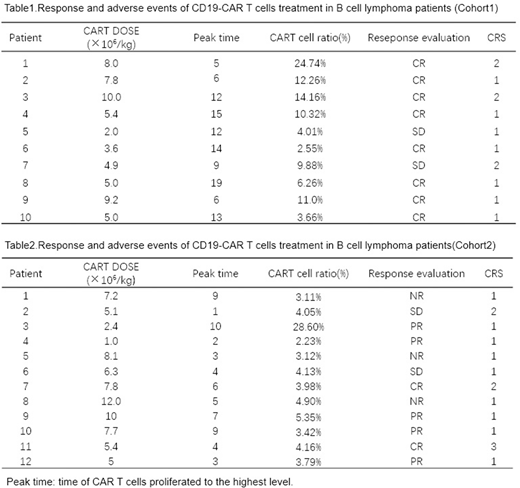
Patients and Methods: This study enrolled B cell lymphoma in two cohorts. Cohort1 includes 10 patients with MRD persistence or reappearance after induction and consolidation therapy or patients acquired partial remission(PR). Cohort2 includes 12 patients with relapsed or refractory (r/r) B-cell lymphoma were included. We used autologous T cells expressing a CD19-CAR T cells to treat these patients. Patients were monitored for response to treatment, toxic effects, the expansion and persistence of CD19-CAR T cells.
Results: 1. A total of 22 patients with B-cell lymphoma received CD19-CAR T cells, the median dose of CAR-T cells was 5.2×106/kg (2.0~10.0×106/kg). The infusions were safe, and no dose-limiting toxicities occurred. 2.The cohort1 overall response rate was 100%. Complete remission occurred in 8 of 10 patients (80%). The other 2patients with DLBCL were stable after CAR T cells treatment. The cohort2 overall response rate was 75%(9/12). Complete remission occurred in 2 of 12 patients (17%),partial remission occurredin 5 of 12 patients (42%).And another 2 patients got stable disease. 3. CD19-CAR T cells proliferated in vivo and were detectable in the blood of patients. The cohort1 and cohort2 peak time of CAR T cells proliferated was 12(5~19) days and 4.5(1~12) days after treatment respectively. And among peripheral blood cells, CAR-T cells accounted for 10.10% (3.55% ~24.74%)and 4.02% (2.23%~28.60%) of T lymphocytes respectively. 4. The cohort1 patients achieved sustained remissions, and at a median follow-up of 10 months(3 ~18 months). None of all the patients relapsed and the median follow-up time was 10 months (3~18 months). However, 9 of the cohort2 patients who had a response maintain a good condition for 40-90 days. Except for one patient with following hematopoietic stem cell transplantation, the remaining patients developed disease progression in different degrees. 5. Cytokine-release syndrome(CRS) occurred in all patients, which in cohort1 were grade 1-2 CRS and in cohort 2 has one patient developed a grade 3 CRS.
Conclusions: CAR-T cell therapy not only plays a role in the rescue treatment of relapsed and refractory patients, but also has a surprising effect in the consolidation and maintenance of B-cell lymphoma, and it is expected to become a treatment that benefits more patients.CD19-CAR T cells might be more effective in the treatment of MRD+/PR B-cell lymphoma patients than in the refractory or relapse patients. High response rate were observed, with fewer adverse reactions. CAR T treatment in MRD-positive B-cells lymphoma patients may be a therapeutic option to put off the progression to relapse and refractory lymphoma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal